InfoBionic, Inc. today announced that it received 510(k) clearance from the U.S. Food and Drug Administration (“FDA”) for MoMe Kardia, a wireless, remote monitoring system designed to aid physicians in their diagnosis of cardiac arrhythmias in patients with a demonstrated need for cardiac monitoring.
MoMe Kardia is a 3-in-1 device that seamlessly transitions between Holter, Event and MCT modes remotely, streamlining patient monitoring time without delays. In addition, MoMe Kardia leverages a comprehensive cloud-based proprietary platform—the first and only of its kind—to deliver on-demand, actionable data and analytics directly to the physicians. The sleek, lightweight form factor of MoMe Kardia is designed so patients can wear it discretely and manage only one device during monitoring.
The MoMe Kardia Difference
Competitive wireless ambulatory electrocardiogram (“ECG”) monitoring systems are two-unit devices comprising a sensor unit and a handheld unit. Traditionally, the sensor communicates the acquired ECG data to the handheld unit using Bluetooth technology, and the handheld unit then analyzes the ECG data and transmits information to the monitoring center through a cellular modem.
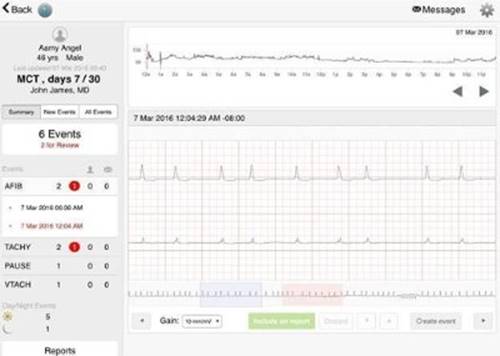
The new generation MoMe Kardia is fundamentally different from these traditional systems. It is the first 3-in-1 single piece device that acquires and stores ECG and motion data and transmits that data via embedded cellular technology to the MoMe Software System, a cloud-based platform with proprietary algorithms for analysis, using the MoMe Device Communications Protocol. The transmitted data is then analyzed by the MoMe Software Platform via completely new and robust server-based algorithms and when indicated, data identified by these algorithms is flagged for physician review (Figure 1). MoMe Kardia requires no patient intervention to capture or analyze data, however it does provide a patient event trigger.
About InfoBionic
InfoBionic is an emerging digital health company focused on creating superior patient monitoring solutions for chronic disease management with an initial market focus on cardiac arrhythmias. For more information please visit www.infobionic.com.